
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY
STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY
Required Report: Required - Public Distribution Date: December 22, 2023
Report Number: AR2023-0019
Report Name: FAIRS Annual Country Report Annual
Country: Argentina
Post: Buenos Aires
Report Category: FAIRS Country Report
Prepared By: Maria Julia Balbi
Approved By: Chase Mcgrath
Report Highlights:
This report is an overview and update on regulations and standards for importing U.S. food and
beverage products to Argentina. Post recommends U.S. suppliers interested in the Argentine market
contact the FAS Buenos Aries office at AgBue[email protected] or (011-54-11 577-4627 or
contact local importers to discuss the rules and regulations applicable to import requirements for specific
products.
2
This report was prepared by the Office of Agricultural Affairs of the USDA/Foreign Agricultural Service
in Buenos Aires, Argentina, for U.S. exporters of domestic food and
agricultural products. While every possible care has been taken in the preparation of this report,
information provided may not be completely accurate either because policies have changed since its
preparation, or because clear and consistent information about these policies was not available. It is
highly recommended that U.S. exporters verify the full set of import requirements with their foreign
customers, who are normally best equipped to research such matters with local authorities, before any
goods are shipped.
FINAL IMPORT APPROVAL OF ANY PRODUCTS IS SUBJECT TO THE IMPORTING COUNTRY’S
RULES AND REGULATIONS AS INTERPRETED BY BORDER OFFICIALS AT THE TIME OF
PRODUCT ENTRY.”
Table of Contents
Executive Summary ................................................................................................................................................ 3
Section I. Food Laws ............................................................................................................................................... 3
Section II. Labeling Requirements .......................................................................................................................... 5
Section III. Packaging and Container Regulations ................................................................................................ 10
Section IV. Food Additive Regulations ................................................................................................................. 13
Section V. Pesticides and Other Contaminants ................................................................................................... 15
Section VI. Other Requirements, Regulations, and Registration Measures ........................................................ 15
Section VII. Other Specific Standards ................................................................................................................... 20
Section VIII. Trademarks, Brand Names, and Intellectual Property Rights .......................................................... 22
Section IX. Import Procedures ............................................................................................................................. 22
Section X. Trade Facilitation ................................................................................................................................. 24
Appendix I. Government Regulatory Agency Contacts ........................................................................................ 26
Appendix II. Other Technical Import Contacts ..................................................................................................... 26

3
Executive Summary
Argentina, the second largest country by land and third largest by population in South America, is
estimated to have the fifth highest GDP per capita in Latin America (Source: Statista). The capital,
Buenos Aires, and its surrounding suburbs, account for nearly one-third of the country’s population.
Alberto Fernando assumed the Presidency in December 2019, and President-elect Javier Milei and his
administration will take over on December 10
th
, 2023.
In calendar year (CY) 2022, the United States exported $213 million dollars in food and agricultural
products to Argentina while Argentina exported $2.5 billion to the United States. Major U.S. exports to
Argentina include planting seeds, dextrins, peptons and proteins, other intermediate products, bakery
goods, cereals, pasta, chocolate and cocoa products, distilled spirits, milled grains and products, and
essential oils.
The Argentine Food Code (Código Alimentario Argentino – CAA) is the guiding legislation for
production, processing, and marketing of both domestic and imported food and beverage products. The
CAA also incorporates regulations and standards agreed upon within Mercosur, a South American
trading bloc which includes Argentina, Brazil, Paraguay, and Uruguay. In June 2019, Mercosur and the
EU announced an agreement on a Free Trade Area which is still pending ratification by EU member
countries. In December 2020, the Fernandez administration signaled a desire to proceed with
implementation, but no progress has been made so far and the direction of the incoming administration
remains to be seen.
Three government agencies regulate food and beverages in Argentina, namely:
SENASA/SAGyP – National Service of Agricultural Food Health and Quality (Secretariat of
Agriculture, Livestock, and Fisheries): animal and plant products and by-products, fishery and seafood
products.
INAL/ANMAT/MS – National Food Institute/National Administration of Drugs, Food Products, and
Medical Equipment (Ministry of Health): processed food and beverages, except wine.
INV/SAGyP: National Wine Institute (Secretariat of Agriculture, Livestock, and Fisheries).
Section I. Food Laws
Framework
The Argentine Food Code - Código Alimentario Argentino (CAA) establishes the regulatory framework
for the production, processing and marketing of both domestic and imported food and beverage products
(F&B). Its primary goal is the protection of public health and maintaining consumers’ confidence in the
safety and quality of food products distributed within Argentina.

4
Established by Law No. 18.284/1969 and enforced by Decree No. 2126/1971, the CAA has over 1,400
articles divided into 22 chapters with technical regulations that establish sanitary and commercial
identification provisions for domestic and imported food products. The CAA is implemented under the
guidelines of Argentina’s national food inspection system - Sistema Nacional de Control de los
Alimentos (SNCA), under whose framework the Secretariat of Agriculture, Livestock, and Fisheries
(SAGyP) and the Ministry of Health (MS) enforce the CAA standards. The CAA is updated by joint
resolutions from SAGyP and MS. To access the CAA please refer to this link.
The National Food Commission (CONAL), an advisory body with representatives from the MS and
SAGyP as well as private sector and consumer organizations, provides support to the SNCA. Provincial-
level food regulatory agencies are also invited to participate in CONAL.
As a member of the Southern Cone Common Market (Mercosur), the CAA incorporates standards
agreed upon within Mercosur, which are influenced by standards from the EU, Codex Alimentarius, and
the U.S. Food and Drug Administration (FDA).
Regulation
Three government agencies regulate F&B in Argentina, namely:
SAGyP, through SENASA, is responsible for governing:
- fresh, chilled, frozen, and thermal-processed products and by-products of animal, plant and seafood
origin.
- mixed canned products (with animal and/or vegetable-origin content) containing over 60 percent
ingredients of animal origin.
- food preparations containing over 80 percent ingredients of animal origin.
SAGyP, through INV, regulates the production and distribution of wine.
The MS, through INAL, within the ANMAT, regulates consumer-ready food products, health
supplements and alcoholic and non-alcoholic beverages, excluding wine.

5
Section II. Labeling Requirements
Chapter 5 of the Argentine Food Code, Articles 220-246, and Resoluciones Mercosur No. 26/2003 and
No. 46/2003 provide the requirements for the labeling and advertising of food products. Resolución No.
26/03 defines labelling as ‘any inscription, image or descriptive or graphic material that has been
written, printed, marked, embossed or otherwise attached to the food package.” Back-of-pack nutritional
labeling is mandatory for products in Argentina even in the absence of a nutritional or health claim.
Animal-Origin Products: Labels for animal-origin products must be submitted to SENASA for pre-
approval prior to entry. An importer may submit a label to SENASA for pre-approval independently or
as part of the import license application. SENASA provides label status feedback directly to the
importer. A label must be affixed to the product prior to domestic distribution in Argentina.
Product specific labeling information for exports of U.S. fresh, chilled, frozen and thermo-processed
products of animal origin are available at USDA's Food Safety and Inspection Service Export Library
page for Argentina.
Non-Animal Origin Processed Foods: Labels for other food and beverage products (see specific wine
requirements below) must be submitted to INAL for pre-approval. Non-animal origin processed foods
may be imported in their original packaging. If the original package label does not include the following
information in Spanish, a sticker label in Spanish must be affixed to the retail package with the
following information:
Name (approved by INAL) and brand of the product.
Identification of origin.
Composition: ingredients in decreasing order of weight, and additives at the end of the list.
Net weight or measure.
Lot number.
Expiration date.
Manufacturer’s name and address.
Importer’s name and address.
Importer’s National Register of Establishment number (RNE).
National Register of Food Product number (RNPA).
Storage, preparation and usage instructions when required.
Nutritional information.
Wine:
Bottled Wine
Imported bottled wines may have printed labels in foreign languages but, for commercial distribution,
they must supplement the information provided with the following additional requirements in Spanish:
6
The importer´s name, address, and registration #.
The product´s legal name must be included in a clear and highlighted way and must not be
smaller than 1.50 millimeters (1.50 mm) high.
The tags and boxes containing bottles and/or packages must have a printed indication key which
identifies the lot number determined by the producer and/or wine bottling establishment. The key
code will be preceded by the letter “L”.
Bulk Wine
Imported bulk wines intended to be bottled in Argentina must include the following information
in their identifying tag: Country of origin.
Font required: No less than three millimeters (3 mm) high, highlighted, horizontal, parallel to the
base of the package/bottle/demijohn, and separated from other text on the label. When the bulk
wine is shipped in bottles and demijohns, the denomination must be listed in all fixed elements
affixed to the label.
For carton, poly-laminated, and bag-in-box packages, the denomination must be printed on the
two (2) largest and most visible sides of the package.
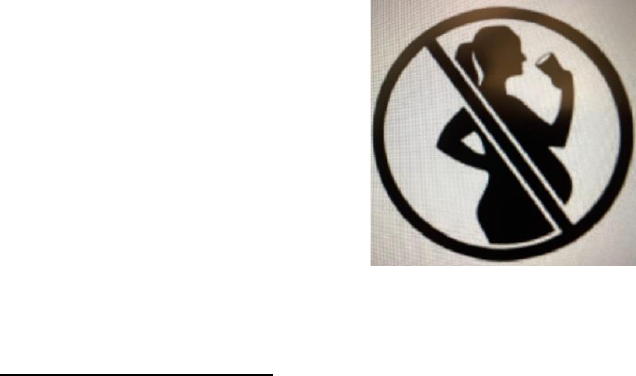
On May 18, 2021, Joint Resolución No. 18/2021 was published in the Official Bulletin incorporating
Article 236 on labeling of alcoholic beverages. It establishes that all labels on alcoholic beverages sold
in Argentina must include a pictogram which consists of a circle with a crossed bar on the shape of a
pregnant person (see below). A period was granted for companies to adjust to this new measure and the
standard will not be implemented until May 19, 2024.
Other Specific Labeling Requirements
Expiration Date Information
Per CAA, the date duration on a label may be provided in any of the following ways:
Best before …
Valid up to …
Validity …Val … (short form of validez, in Spanish)
Is due …
Expiration date

7
Vto. (short form of vencimiento, in Spanish)
Venc. (short form of vencimiento, in Spanish)
Preferably best before …
Expiration dates are to be used on all products except for:
Fresh fruit and vegetables, including potatoes which have not been peeled, cut, or treated in a
similar way
Wines, liqueurs, sparkling wines, flavored wines, fruit wines, and sparkling fruit wines
Alcoholic beverages that contain 10 percent or more alcohol.
Bakery and pastry products which, by the nature of their content, are usually consumed within 24
hours after manufacturing
Vinegar
Solid sugar
Confectionery products which consist of flavored or colored sugars, such as candy
Chewing gum
Food quality salt (does not apply to enriched salts)
Food products which have been exempted by specific Mercosur Technical Regulations.
Nutritional Claims
Article 235 Fifth of CAA (Joint Resolución No. 40/2004-SPRRS and No. 298/2004-SAGPyA) with
Resolución No. 46/03 regulates nutritional labeling of consumer-ready packaged foods that are produced
and marketed within Mercosur. Unless otherwise specified, nutritional labeling of packaged foods must
include the following information (in Spanish, for imported products):
Name of the product
List of individual ingredients
Net Contents
Country of Origin
Name and address of the importer
Lot/batch number, expiration date and preparation and usage instructions, when relevant.
The label should be on the main face of the product, with the name of the product, its quality, in
contrasting colors to insure visibility. The size of the letters and numbers must be, except for the net
content listing, no less than 1 mm.
Prebiotics and Probiotics
Prebiotics
The denomination of Food with Prebiotics refers to a food product that includes an added authorized
prebiotic. The product will be sold in a package that has proven to be safe and whose label indicates the
specific identification of its component/s. The product will be labeled: “… with prebiotics,” by filling in
the blank with the sales name of the food product. Food products manufactured with prebiotics will be

8
authorized once a satisfactory in vivo trial evaluation shows the functionality assigned to it or its
prebiotic component/s. The evaluation is conducted by an Evaluation Committee composed of
specialized professionals from the Sanitary Authority or assigned by them on a case-by-case basis.
Probiotics
The denomination of Food with Probiotics refers to a food product with a charge of feasible cells
between 106 and 109 CFUg (colony-forming unit/gram) during its period of minimum duration. The
product will be sold in a package proven to be safe, and whose label indicates the specific identification
of its strains and the concentration of feasible cells of each strain (UFC/g). The product will be labeled:
“… with probiotics,” by filling in the blank with the sales name of the food product.
Food products manufactured with probiotics will be authorized once a satisfactory in vivo trial
evaluation confirms the functionality assigned to its probiotic strain(s). That evaluation will be
performed by an Evaluation Committee composed of specialized professionals from the Sanitary
Authority or assigned by them on a case-by-case basis.
Known Allergens on Prepackaged Foods
Joint Resolución No. 57/2010 and No. 548/2010 requires a declaration on an ingredient label if a trace,
or any of the allergenic substances listed below, are present in a food as an ingredient. This information
must be presented in contrasting colors to ensure visibility and include the following: “Contains ...”
followed by the name of the substance and/or “Traces of …”
1. Cereals, i.e., wheat, rye, barley, oats, and hybridized strains and products thereof;
2. Crustaceans and products thereof;
3. Eggs and products thereof;
4. Fish and products thereof;
5. Peanuts and products thereof;
6. Soybeans and products thereof;
7. Milk and products thereof (lactose included);
8. Tree nuts and products thereof; and
9. Sulphur dioxide and sulfites in concentrations of more than ten milligrams per kilogram (10mg/kg).
In October 2017, Argentina introduced new allergen legislation which corresponds to the Codex list,
except Argentina uses “Cereal, i.e. wheat, rye, barley, oats, or their hybridized strains and products of
these – without reference to gluten (Joint Resolución No. 11-E/2017).
Precautionary Allergen Labeling
The declaration of the allergen substance must be stated as follows (filling in the blank with the name of
the appropriate substance/s):
“Contains …,” or
“Contains traces of …,” or
“Contains … and traces of …”

9
When there may be the possibility of accidental cross contamination during the manufacturing process,
the precautionary language must be included in the label as follows:
“May contain …”, or
“May contain traces of …,” or
“May contain … and traces of …”
To request authorization for the use of the precautionary phrase, the company must submit to INAL a
sworn statement including the following language “that even having used GMP, there exists the
possibility that there has been accidental cross contamination during the manufacturing process due to
“…” providing the appropriate justification for the incident.
Organic Products
Per Decree No. 206/2001, imported products labeled as "organic" must originate from a country whose
organic standards have been approved by SENASA as equivalent to Argentina’s. Otherwise, prior to
export, imported organic products must be certified by an Argentine certifying agency approved by
SENASA. The US National Organic Program has not yet been determined to be equivalent by
SENASA.
Natural
In December 2020, CONAL announced that it will no longer permit the use of the term “natural” on
food labels except when the sales denomination specifies it in the CAA. The term “natural” is currently
regulated in natural flavorings or artificial flavorings identical to natural flavorings, natural yogurt,
natural fermented milk, natural acidophilic milk, natural Kefir, natural Koumiss, natural curd, fish
preserves, and natural mineral water.
Biotech Products
Biotech foods in Argentina do not have specific labeling requirements.Salt
Law No. 26.905/2013, “Sodium Intake – Maximum Values,” requires the inclusion of warning messages
about the health risks of a high-salt intake for consumers in meat products, flour products, soups,
dressings, and pickled vegetables.
Front-of-Package Labeling (FOPL)
Argentina’s FOPL draft bill, which had already been approved by the Senate in 2020, was passed on
October 28, 2021, by the Chamber of Deputies and was signed into law shortly before the end of the
Argentine legislative session. In September 2022, it was incorporated to the CAA through Joint
Resolución No. 7/2022 as the “Law of Promotion of Healthy Food.” It requires that manufacturers of
processed food products adopt a FOPL model based on black octagons to warn of excess levels of sugar,
sodium, calories, and total and saturated fat. It also includes FOPL prohibitions on professional
endorsements, and publicity and advertisements targeting children. In addition, the new FOPL policy is
being discussed at the Mercosur level for harmonization among associate members, which could lead to
further amendment.
10
Section III. Packaging and Container Regulations
Generally, Argentina does not impose special packaging or container-size requirements by product,
however, some products, such as salt, do have specific requirements. Exporters should always make sure
that their importing partners are aware of the latest changes in Argentine regulations.
Chapter IV of the CAA, Articles 184 and 185, establishes the requirements for protecting food
containers from external agents of alteration, contamination and adulteration from manufacturing and
processing to consumer use.
Per Article 186, the following materials are allowed:
Stainless steel, cast iron or whipped iron, coated or not with technically pure tin and chromed tin.
Copper, brass or bronze covered entirely by a layer of gold, silver, nickel, chrome or tin
technically pure, except for the requirement of coating the boilers, vessels and pans for cooking
of sweets and syrups, mortars, scale plates and dumbbells.
Tin, nickel, chromium, aluminum and other technically pure metals or their alloys with harmless
metals.
Tin plate of first use.
Ceramic materials, baked clay glazed internally that does not yield lead or other compounds
harmful to the acid attack: glass, marble and odorless woods.
Kitchen utensils of diverse metals, with antiadhesive coating or pure polytetrafluoroethylene
(Teflon, fluon, etc.). Vegetable, animal, or synthetic fiber materials, waterproofed or not with
harmless materials.
Different types of films based on regenerated cellulose authorized for packaging of food
products in general. Must declare the exact composition of the films, analytical verification, and
final approval by the health authority.
Iron enameled or enameled that does not yield lead or other harmful compounds by acid attack.
And the use of:
Galvanized or galvanized iron.
The internal lining of containers, tubers, utensils, or other elements with cadmium.
The materials (metals, plastic materials, etc.) which may yield to food, metal, or metalloids in a
higher proportion than those established in Article 156.
According to Article 186 bis, which focuses on paper and cardboard, the packing and cellulose
equipment referred to in this Article shall be manufactured following good manufacturing practices,
compatible with its use for direct contact with food. Only substances included in the “Positive List for
Containers and Cellular Equipment in Contact with Foods,” and the “Positive List of Resins and
Polymers for Containers and Plastic Equipment” may be used for the manufacturing of the containers to
which this document refers.
11
According to Article 186 Annex A, substances approved in the most recent official documents of the US
FDA and/or German BGA and/or Italian legislation of the EEC, may also be incorporated into the
Positive List.
Positive List for packaging and cellulose equipment in contact with food
- 1. Fibrous Raw Materials
- 2. Non-Fibrous Materials (Mineral Cargo)
- 3. Auxiliary Substances:
- 3.1 Internal and superficial bonding agents
- 3.2 Retention and drainage agents
- 3.3 Dispersing and flotation agents
- 3.4 Antifoaming agents
- 3.5 Antimicrobial agents
- 3.6 Conservatives
- 3.7 Aluminum sulfate
- 4. Special Paper Improvers
- 4.1 Agents that improve the mechanical properties of wet paper
- 4.2 Moisture retention agents
- 4.3 Optical Coloring and bleaching materials
- 4.4 Coating agents and surface improvers
Article 185 tris. covers the general provisions for regenerated cellulose films in contact with food.
Container Registration
Resolucion General AFIP No. 3615/2014 established a container information system through a web-
based database, Registry of Containers. This system, which is applicable to both imports and exports,
provides the Argentine government with container specific information that can be used to monitor and
control container-based trade.
Packaging Sustainability Measures
At the national level, Argentina’s Ministry of Environment has developed the "Estrategia Nacional de
Consumo y Producción Sostenibles" (National Strategy of Sustainable Consumption and Production),
which includes the following law and strategy:
National Law No. 27.454/2018 – “Plan Nacional de Reducción de Pérdidas y Desperdicio de
Alimentos” (National Plan of Food Loss and Waste Reduction)
This law was created in 2018 by the Ministry of Agriculture, Livestock, and Fisheries, and it aims to
propose and implement public policies, based on consensus with different sectors, that would reduce
food loss and waste. In line with that goal, National Law No. 25.989 was passed to clarify food donation
rules, which are within the jurisdiction of the Ministry of Health.
"Estrategia Nacional para la Gestión Integral de Residuos Sólidos Urbanos" (National Strategy
for the Management of Solid Urban Waste).

12
The Ministry of Environment prepared this national strategy focusing on public health, environmental
preservation, a significant reduction of waste and, finally, the disposal of solid urban waste in a
sustainable way, eradicating and ultimately, closing open-air landfills.
At the municipal level, the Government of the City of Buenos Aires enforced Law No. 1854/2005 for
“Gestión Integral de Residuos Sólidos Urbanos” (Integral Management of Solid Urban Waste), whose
main goal is to establish principles, obligations, and responsibilities for the management of solid urban
waste within the City of Buenos Aires in an appropriate sanitary and environmental way, to protect the
environment, human beings, and goods. In that regard, the concept “Zero Waste” was adopted. Decree
No. 760/2008 further defines concepts and terms included in Law No. 1.854.
More relevant Chapters and Articles of Law No. 1854/2005:
Chapter II
Article 8 promotes the following:
1. Reduction of waste and use of long-lasting or re-usable products.
2. Product recycling and sorting of products that can be recycled.
3. Sorting and composting and/or biodigestion of organic waste.
4. Measures towards the gradual replacement of disposable for reusable packaging and sorting of
packaging and bottles to be collected separately by companies that use them.
Article 9 establishes standards for the producer, importer, distributor, agent, or any other person
responsible for placing a product in the market, which after use will become waste. That person will
have the following obligations:
1. Manufacture products or use packages or bottles which minimize waste and facilitate reusing or
recycling and allow disposal which is less harmful for human health and the environment.
2. Take charge of the waste management derived from his/her products, or participate in an organized
system of waste management, or contribute to a public waste management system.
Chapter XI
Article 40: In any of the methods of public procurement, any organization within the City of Buenos
Aires must give priority to those products which have been produced using recycled or reused inputs.
Chapter XVIII
Article 59: As of the date of implementation of this law, it is mandatory that solid urban waste is placed
in biodegradable bags.

13
Section IV. Food Additive Regulations
Chapter XVIII of CAA contains a positive list of authorized food additives (FA) in Article 1400 which
incorporates CODEX-approved additives. In addition, additives used in food product imports must also
be on the positive list maintained by Mercosur. If the additive in question is not on this list, an
application for registration must be submitted to CONAL.
The following are key points under Chapter XVIII of the Code which summarizes the scope of food
additive regulations and laws that are harmonized within Mercosur:
a) FA must be safe or through their action as additives.
b) FA must be included in the CAA positive list.
c) FA must be exclusively used in food products included in CAA.
d) FA must comply with CAA requirements related to designation, composition, identification, and
purity.
e) FA must not be used to deceive the consumer.
f) FA quantity added to a food product must be the minimum quantity necessary to minimize any
potential danger to consumers’ health.
g) FA must be sold in closed original packages.
h) A legend stating “Exclusive Industrial Use (Uso Industrial Exclusivo)” with letters no smaller than 50
percent of the FA denomination and clearly visible must be placed on the label right below the
denomination.
i) All authorized FA added to a food product must be included in the label of such product through
language that indicate the types of FA used, such as: Authorized Antioxidant (Antioxidante Permitido),
Authorized Emulsifier (Emulsionante Permitido), etc. In addition, food products which contain
tartrazine, benzoic acid (or its calcium, potassium, or sodium salts), and sulphur dioxide (and
derivatives) must be declared on the product label.
Through the FA transference principle, CAA establishes that all FA used in raw materials or other
ingredients (including FA) which have been transferred to a food product will be exempt from the
declaration in the list of ingredients, under certain conditions, as stated in Mercosur/GMC/Resolucion
No. 105/94 – Principio de Transferencia de Aditivos Alimentarios (Transference Principle of Food
Additives).
Registration of Food Additives
Food Additives Used in Products of Animal Origin (registered at SENASA)
Main Documentation Required:
- Registration form of adjuvant additives, packages, and other related products.
- Product technical specifications.
- Technical specifications required by the company which owns the product to be authorized for each of
its individual components.
- Product protocols and trial reports authenticated by the Foreign Relations Ministry.
- Company’s and processing establishment’s RNEs.

14
- INAME-ANMAT Approval certificate (for hand washing products).
- Valid label, and label used in the country of origin.
- Product formula or monograph.
Food Additives Used in Products of Vegetable Origin (registered at SENASA)
Main Documentation Required:
- Letter with letterhead indicating the intention of additive registration.
- Registration form.
- Copy of CUIT (tax identification number)
- Payment of fee.
Presentation of Information for Registration of FA and Technology Ingredients
For active ingredient/s of the FA or ingredient technology accepted by international, regional, and/or
national standards:
- Specify what international and/or national regulatory organizations have registered or partially
evaluated active ingredients of FA or ingredient technology that will be registered. And under what
number such active ingredients have been registered.
- Full technical, scientific and/or common name that identifies active ingredient/s.
- Last date of evaluation of active ingredient/s by international, national and/or regional regulatory
organizations.
- Proposed technological function/s.
- Food product/s for which their use and doses are proposed.
- ADI for food product/s for which their use is proposed.
- Monograph of the manufacturing process: (1) flow diagram of the manufacturing process, (2) specify if
active ingredient/s is/are obtained from raw material/s of natural origin, or of chemical or biotechnical
synthesis.
- Degree of purity of the FA or ingredient technology.
- Contaminants present in the FA or ingredient technology, including quantitative specification.
- Qualitative and quantitative analytical methodology proposed for determining active ingredient/s and
elements of accompanying (contaminant) substances.
For registration of Food Additives used in processed foods, see below, Section VI – INAL.
On February 9, 2021, Joint Resolución No. 12/2021 was published in the Official Bulletin amending
Article 1398 of the CAA with the intention of updating specifications for additives using criteria that
had been developed and adopted by international organizations such as FAO/WHO, JECFA (Joint
Expert Committee on Food Additives), and FCC (Food Chemicals Codex).

15
Section V. Pesticides and Other Contaminants
SENASA Resolución No. 934/2010 establishes requirements that must be met by agricultural products
and byproducts for domestic consumption. A summary of the main points is provided below:
Article 1 – Products for domestic consumption: Products and by-products which are imported or
produced locally for domestic consumption must comply with national maximum residue levels (MRLs)
established in Annex I of the present resolution. Products and by-products not included in Annex I must
comply with a default value of 0.01 mg/kg equivalent to the detection limit of the analytical method.
Article 2 – Products not traditionally grown in the country: Those imported agricultural products
and by-products that are not traditionally grown in the country, and for which a national MRL of the
active ingredient has not been established, will be allowed entry only if there is an MRL approved by
Codex Alimentarius, and if the risk evaluation to the consumer carried out by the CSA (Competent
Sanitary Authority) does not indicate unacceptable risks.
Article 3 – Residues of prohibited compounds: For those residues of compounds which are persistent
in the environment and were used as pesticides but are no longer registered as such and can cause food
contamination, the values set up by Codex Alimentarius will be adopted as extraneous MRLs.
Section VI. Other Requirements, Regulations, and Registration Measures
Facility and Product Registration Requirements
SENASA: Animal Products, By-Products and Derivatives
In compliance with Decree No. 4238/1968 (updated September 2018) – “Requirements for the
Inspection of Animal Products, By-Products, and Derivatives Related to the approval of Animal
Products, By-products, and Derivatives that are Manufactured or Used in Approved Establishments,” all
imported food products must be registered with SENASA prior to importation.
For product registration, SENASA requires a company-produced document that provides the descriptive
aspects of the products being submitted for registration (monograph). U.S. producers may file the
document directly with SENASA or by an importer as part of the import license application. Based on
the descriptive characteristics of each product in the document, SENASA assigns each product a unique
registration number that becomes a component of its product label. Please note: for all animal products,
including fishery products, no U.S. government sanitary authority signature is required on a company
document submitted for product registration to SENASA. After a product is registered and receives its
unique identification number, any Argentine importer(s) may apply for an import permit from SENASA
for a registered product.
SENASA requires additional product information that may not be provided through the registration
document, FSIS Forms 9060-5 or 9060-7 and Letterhead Certificate to comply with sanitary import
requirements.
16
SENASA only accepts animal products from FSIS-approved facilities. SENASA reserves the right to
prior inspection and approval of the establishments of origin by a SENASA official, when deemed
necessary.
Further information is available on the FSIS Export Library at:
https://www.fsis.usda.gov/inspection/import-export/import-export-library/argentina
SENASA: Plant Products
U.S. plant product imports must have a USDA Phytosanitary Certificate signed by an APHIS
representative and an import certificate (AFIDI) issued by SENASA. The importer declares the import
product characteristics (product name, destination, and origin) to SENASA which then grants an AFIDI
which specifies the type of sanitary certificate needed for importation. The exporter normally provides
this information to USDA/APHIS to obtain the appropriate certificate.
SENASA holds the product at the port of entry for inspection and verification of the requirements as
stated in the AFIDI. Once verified, SENASA issues an import certificate for Customs that allows the
release of the product.
For more information on this certificate process, please contact the USDA/APHIS Regional
representative:
Fred Wang, Area Director
USDA APHIS/International Services
U.S. Department of Agriculture
Lima, Peru
On January 30, 2019, SENASA Resolución No. 76/2019 removed the Registry of Importers and
Exporters of animals, plants, reproductive and/or propagative material, products, by-products and/or
derivatives of animal or plant origin or merchandise which contain ingredients of animal or plant origin,
from SENASA jurisdiction. As needed, SENASA will obtain information on importers/exporters from
Customs’ Registry of Importers and Exporters within the Federal Administration of Public Revenue
(AFIP).
New market importers/exporters must register one time, on-line, with Customs/AFIP before beginning
operations in Argentina. All importers/exporters must receive a tax identification number (CUIT), which
serves as their registration number. CUITs are required to conduct many types of business operations in
Argentina, by both foreign and domestic companies.
Cannabis and Industrial Hemp
In August 2022, the “Strategic Table for Cannabis and Industrial Hemp” was created by SENASA
Resolución No. 454/2022 to strengthen the productive development of cannabis and industrial hemp in

17
Argentina, and to coordinate actions with other government organizations with jurisdiction over this
crop, primarily the National Seed Institute (INASE, in Spanish).
In addition, Law No. 27.669 provides the regulatory framework for the development of industrial hemp,
including production and marketing, both for domestic consumption and export, and scientific research
of the cannabis plant, seeds and products destined for therapeutic and industrial use.
Cannabis Seed Import
Besides complying with SENASA import requirements for any plant products through the request of an
import permit (AFIDI – see above), cannabis seed imports are regulated by Law No. 27.350/2017.
An import permit must be requested to SENASA attaching the Certificate of Variety Register, which is
issued by INASE, and approval of the Research Project by the Ministry of Health.
For additional information: [email protected]
INV: Wine
Argentine requirements for the import of wines and musts are as follows (Law No. 14.878):
Registration:
- The importer must be registered at INV. He/she obtains a registration number to control the
importation process and make consultations related to that import operation or future importations.
- The importer must be registered at AFIP to initiate the registration process.
- A “Registration Certificate” is issued to the importer within 24 hours at no cost.
- Required documentation:
The importer must submit to INV, two copies of the “Registration Request as Importer of Wine
Products,” together with a copy of AFIP registration statement. All original copies of documents
must be signed, including printed names. Once documentation is verified, a “Registration
Certificate as Importer of Wine Products” is issued.
Import Procedure:
- The importer must complete a “Unique Import Guide,” considered a sworn statement, informing INV
about wine and must imports, and requesting controlled sampling. The statement serves as a safeguard
for the transportation of imported products from Customs to the receiving wineries or must processing
facilities. The guide includes the following information: importer´s business name, registration #,
address, name and address of the warehouse where the merchandise is stored, country of origin,
Customs at Port of Entry, date of entry, category #s, product type/s, harvest year, variety, quantity and

18
type of bottles, liters, # of analysis at origin, C.I.F. value, identifying key code, place and date of
submittal, importer´s and government official´s signature and stamp.
- Products to be imported must comply with limits of analytical composition required by similar
domestically manufactured products.
The “Import Guide” must be submitted at least 48 hours before the declared date of entry.
Imported bottled wine products may have a country-of-origin tag affixed to the bottle, and it
must include the following information (either in the tag or in an additional label): Wine type, as
classified in Argentina (fine wine, table wine, sparkling wine, etc.)
Color (red, white, rose).
Sparkling wines are classified in relation with the sugar content (nature, extra brut, brut, sec,
demi sec, dulce).
Country of origin.
Importer´s name and address.
Importer´s INV registration #.
Packaging capacity in cl, ml, or cc.
Alcoholic content (% by volume)
Analysis # of Free Circulation.
Legend: “Beber con moderación” and “Prohibida su Venta a menores de 18 años.”
Note: Argentina’s Law No. 14.878 - Art. 22/1959) states that bulk imported wine products are not
allowed to be mixed with other imported wines or with domestically produced wines.
- Required documentation:
The importer must submit three original copies of the “Import Guide” at INV.
He/she must also submit the original copy of the analysis of origin of each of the imported
products, issued by official laboratory (or authorized laboratory by CSA of the country of origin.
Once all documentation is verified, the importer obtains two copies of the “Import Guide,” which
will be included in the Customs documentation package.
Finally, the importer must submit a Customs document entitled “Import for Consumption.”
INAL: Processed foods, and alcoholic and non-alcoholic beverages, except wine
Processed products and alcoholic and non-alcoholic beverages, except wine, require product registration
with INAL prior to importation by a registered importer. The registration requirements for imported
processed foods are listed below. An importer registered with the National Register of Establishments
(RNE) applies for a National Register of Food Product Number (RNPA) with the following information:
Letter announcing intention to register the product
RNPA Application form
Flow chart and document detailing the product manufacturing process, shelf-life, product
specifications, shipping and storage requirements, quality controls, and packaging type
List of ingredients and additives

19
Original label and three copies
Supplementary label and three copies
Certificate of Free Sale and fit for human consumption issued by the official sanitary authority of
the country (or state) of origin, or guaranteed by the State Chamber of Commerce. Please note:
no Certificates of Free Sale issued by manufacturing companies/exporters/importers and
endorsed by official sanitary authority or State Chamber of Commerce will be accepted.
Payment of fee
For food additives, technology adjuvants, and raw materials, tests must be submitted as per CAA
requirements.
Copy of the importer’s RNE.
Once the RNPA has been issued and the product is at a port of entry, the importer obtains a Certificate
of Free Circulation at INAL. The requirements are listed below:
Letter requesting a Certificate of Free Circulation for the product/s
Shipment information
Copy of the invoice
Bill of lading
Copies of the RNE and RNPA plus approved label
Manufacturing date and shelf life
Sanitary Certificate/Fit for Human Consumption Certificate (including lot #, invoice #, issued by
the Competent Sanitary Authority – electronic signature not accepted.)
Certificate of aging (for alcoholic beverages, except wine), issued by CSA (Competent Sanitary
Authority).
Once the importer has an RNPA for a given product, it is not necessary to register the product again for
subsequent importations. However, a Certificate of Free Circulation for each shipment is required.
Per Resolución No. 876/1997, consumer-ready food products from Mercosur countries (Brazil,
Paraguay, and Uruguay) are not subject to the registration process, except for certain specific products.
An importer purchasing food products in Mercosur countries must submit a sworn declaration with the
following attachments: free circulation/fit for human consumption certificate, issued by the sanitary/food
safety authority of the country of origin; numeric identification (if applicable); original labels; lot
number/s; total weight; and, in those cases when the exporter is not the manufacturer of the food product
being imported, a certificate signed by the manufacturer stating that he/she is aware of the export
operation to Argentina.
In the case of health supplements, the statements mentioned above also apply with slight differences.
Instead of the RNE, importing establishments need to obtain from INAL a National Register of
Establishment of Health Supplements number (RNESD). And instead of the RNPA, a National Register
of Health Supplements number (RNSD) is needed. The requirements are as follows:

20
Request register authorization at INAL
Each presentation must be signed by the owner of the product, the local legal representative, and
the technical director of the local establishment.
Certificate of Free Sale from the country of origin, issued by the national or state sanitary
authority, stamped by the Argentine Consulate, or certified by The Hague Convention Apostille.
Analysis of the product for verification that it complies with CAA standards.
The Argentine importer must have a technical director who will be responsible for: the genuine
origin of the product, the legitimacy of the document, the shelf life of the product, the quality
control of the shipment, the correct labeling, and the appropriate "warning" literature on each
package or promotional material, when required.
Other Certification and Testing Requirements
U.S. products and by-products of animal origin imports must originate from U.S. plants approved by the
United States Department of Agriculture and the Food and Drug Administration and must be
accompanied by an official health/sanitary certificate. While SENASA accepts products from any
FSIS/FDA-approved facility, it reserves the right to prior inspection and approval of the establishments
of origin, when deemed necessary. All U.S. meat plants exporting products and by-products of animal
origin to Argentina may be audited by SENASA (See SENASA Resolución No. 816/2002 ).
Section VII. Other Specific Standards
Trans Fatty Acids Limits
SAGyP and Secretariat of Health Quality Joint Resolucion No. 16/2023 in Article 1 states as follows:
Article 155 tris of CAA is hereby replaced by the following text: “Article 155 tris: the content of trans
fatty acids in industrial food processing, including those used as ingredients and raw materials, must not
exceed 2 percent of total fat content. These limits do not apply to fats originated from ruminants,
including dairy fat. The use of partially hydrogenated oils and fats in the manufacturing of food,
ingredients, and raw materials is prohibited.”
Health supplements
The MS’s National Administration of Drugs, Food and Medical Devices (ANMAT), through INAL,
regulates health supplements. On December 29, 2020, Joint Resolución No. 3/2020 was published in
the Official Bulletin, which modified Articles 1381 and 1381 tris of the CAA. Besides establishing the
definition of health supplements, the resolution sets up special requirements about labeling and
composition of these types of products.
Enriched Flour
By Law No. 25.630 and Decree No. 597/2003, all flour-based products must be manufactured with
enriched flour, with the exception of diet products, flours destined for the manufacturing of products for
export, flours for export, and organic flours (Law #25127). The required nutrients are as follows:
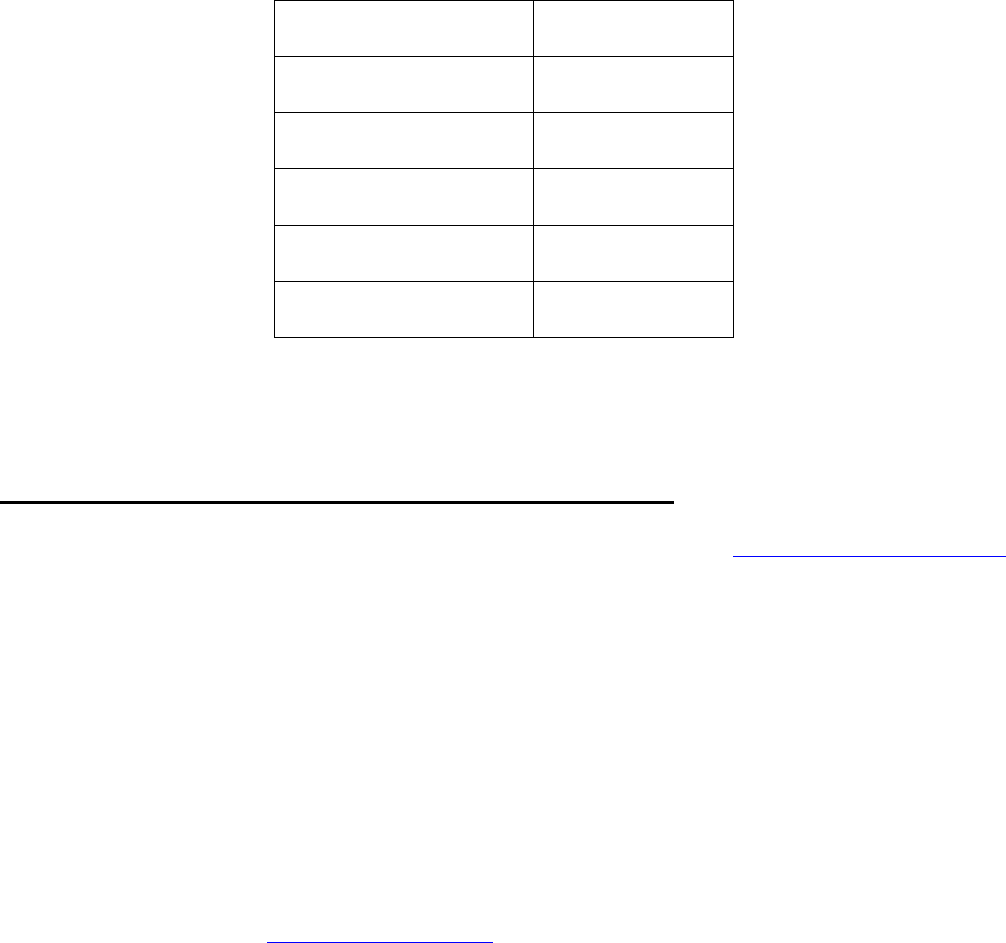
21
Nutrient
Quantity (mg/kg)
Iron
30
Folic Acid
2.2
Thiamin (Vitamin B1)
6.3
Riboflavin (Vitamin B2)
1.3
Niacin
13.0
Labels must show the content of each nutrient, the wording “Enriched Flour, Law No. 25.630 (Harina
Enriquecida, Ley No. 25.630,” in Spanish) and the quantities listed in the table above.
Plant-based Meat/Dairy Alternative Products (Vegan/Vegetarian)
The terms “vegan” and “vegetarian” were incorporated to the CAA through Joint Resolución No. 5/2022
of Argentina’s Secretariat of Health at the Ministry of Health, and the Secretariat of Food Products,
Bioeconomy, and Regional Development at the Ministry of Economy.
Vegan Products
To use the legend “vegan” in food product labeling, an official authorization is required by the
“Evaluating Committee for Authorization of the Use of the Vegan Attribute for Food Products.” That
committee is composed of representatives from ANMAT, through INAL, and SAGyP.
The Evaluating Committee will request supporting documentation justifying the use of the legend
“vegan,” and may also require an on-site audit of the product manufacturing plant to verify the veracity
of the documentation submitted. Laboratory tests may be also requested to supplement verification.
For additional information: [email protected]
Vegetarian Products
In order to obtain authorization to use any of the following legends: “Sólo con ingredientes de origen
vegetal” (Manufactured only with plant-based ingredients), “100% vegetal” (100% plant-based) or
“Alimento vegetariano” (Plant-based product), food manufacturers and importers must be registered at
INAL, and the product in question must be registered as well at the National Register of Food Products
(RNPA, in Spanish).

22
For imported food products authorized by INAL/ANMA to use the “vegan” and/or “vegetarian” legend,
the importer must submit a sworn declaration stating the origin of ingredients used in the manufacturing
of the product (including additives and agents).
Product samples
Products samples with low commercial value (under US$100) are not subject to import duties. Post
recommends that exporters coordinate with importers/agents to obtain a Certificate of Free Sale from
INAL which is required for all samples.
Section VIII. Trademarks, Brand Names, and Intellectual Property Rights
Although Argentina is a signatory, it has not ratified the World Intellectual Property Organization's
(WIPO) Patent Cooperation Treaty (PCT). Therefore, brands and trademarks should consider
registration in Argentina for proprietary protection. For additional information on brand, trademark,
patent, or industrial design registration, please refer to the following website:
https://www.argentina.gob.ar/inpi
Section IX. Import Procedures
Import Authorization System
In 2015, the GOA established the “Sistema Integral de Monitoreo de Importaciones (SIMI),” an
integrated system for import control through non-automatic import licenses (NAIL) or automatic import
licenses (AIL).
However, on October 4, 2022, Argentina’s Secretariat of Commerce published Resolución No. 26/2022
in the Official Bulletin, which adds non-tariff barriers to roughly 2,670 HTS codes, including a wide
variety of agricultural products, and F&B.
This new measure consists of the shifting of many imported products from the AIL system to the NAIL
system. All importers are required to obtain approval from the Central Bank of Argentina to pay for
imports with dollars (instead of Argentine pesos).
During 2023, the GOA significantly cut down overall imports, mostly driven by a decreased availability
of foreign exchange. This reduction was implemented through the utilization of a discretionary system
known as SIRA (Sistema de Importaciones de la Republica Argentina – Import System of the Argentine
Republic).
In addition, Argentine Customs created an “Import Radar,” which consists of a centralized database
where they keep a risk profile for each importer, including their criminal records and their foreign trade
operations so they can verify that the declared price agrees with the market price.
23
Agricultural and F&B products affected by the NAIL system are included in Harmonized Tariff
Schedule chapters below:
Chapter
Product Description
Chapter 2
Meat, offals.
Chapter 4
Dairy, eggs, honey, animal products.
Chapter 5
Other animal products.
Chapter 9
Coffee, tea, mate, spices.
Chapter 16
Preparations of meat, fish, crustaceans, mollusks, other aquatic invertebrates.
Chapter 17
Sugar, confectionery products.
Chapter 18
Cocoa, cocoa preparations.
Chapter 19
Preparations of cereals, flour, starch or milk, bakery products.
Chapter 20
Preparations of vegetables, fruits, nuts, other parts of plants.
Chapter 21
Miscellaneous edible preparations.
Chapter 22
Beverages, spirits, vinegar.
Chapter 23
Residues and waste from food industries, preparations of animal feed (fishery
products, pet food).
Chapter 44
Wood, wood charcoal.
Chapter 51
Wool, fine or coarse animal hair.
Chapter 52
Cotton.
Decree No. 1812/1992 supplements Decreto 2092/1991 regulating imported F&B (both manufactured
domestically and imported), except wine. The main articles of the decree state the following:
Articles 2 and 3 state that sanitary and phytosanitary controls on imports of animal and vegetable
origin not for retail sale will be carried out by SENASA prior to Customs release.
Articles 5 and 6 state that consumer-ready food products that have proven stability and were
registered in CAA will be tested and inspected by INAL only after Customs has released them to
the domestic market. Once the importer has proven to INAL, at the time of registration that the
product has been manufactured, packaged, and transported in accordance with Argentine sanitary
regulations, INAL will issue a certificate of stability authorizing the shipment release from
Customs without the need for inspection.
Article 7 states that either when the importer of a consumer-ready product is unable to show the
certificate of stability, or when the food product has suffered evident damage, INAL has the right
to inspect and test the shipment before it is released from Customs.
Article 8 states that, when there are justified reasons to presume risk for human, animal or plant
health because of the introduction of food products to the country, any of the three above-
mentioned agencies (SENASA, INAL, and INV) has the right to perform inspections to the

24
shipments prior to product entry into Argentina provided that the importer is informed about this
procedure.
Article 10 states that, for all those food products that require previous inspection, the CSA, i.e.
SENASA, INAL, and/or INV, have up to 30 days to issue the free sale certificate.
Article 11 states that Customs will release the consumer-ready food products that have a stability
certificate. In the case of those products requiring a previous inspection, Customs will need
authorization from the CSA to release the shipment.
Article 12 states that, if the CSA does not authorize the shipment release, Customs may allow the
importer to transport the shipment to his/her warehouse. In that case, the product cannot be
marketed until the appropriate certificates are submitted to Customs.
Article 13 states that a random sample from every shipment will be taken by a Customs official
before releasing the shipment.
Article 14 states that, when the importer does not submit the authorization from CSA in the term
established as per Article 10 of this Decree (30 days) due to his own fault, Customs and CSA
will destroy or re-export the shipment and the importer will be liable to a fine, expenses and
penal charges resulting from these procedures.
Article 18 states that, in the case of imported consumer-ready foods, it is considered that CAA
requirements are met when products come from the following countries/regions: Australia,
Austria, Canada, Switzerland, Israel, U.S., Japan, Norway, New Zealand, EU, Sweden, and
countries with specific food safety agreements with Argentina. In all of these cases, the food
products must have been manufactured under the same controls as those products destined for
human consumption in the domestic market of the country of origin.
Container Registration
Resolucion General AFIP No. 3615/2014 established a container information system through a web-
based database, Registry of Containers. This system, which is applicable to both imports and exports,
provides the Argentine government with container specific information that can be used to monitor and
control container-based trade.
Section X. Trade Facilitation
Advance ruling: In January 2018, Argentina ratified the WTO Trade Facilitation Agreement and, under
that framework, it allows advance rulings for the importation and exportation of all types of products
included in the Harmonized Tariffs Schedule Code.
Pre-clearance programs: Currently, there are no pre-clearance programs operating in the U.S. for the
export of fresh agricultural products to Argentina.

25
E-certificates: E-certificates are used by all regulatory agencies in Argentina governing agricultural,
and F&B, namely, SENASA, INAL, and INV. However, some still keep the option of carrying out on-
site administrative procedures. E-certificates are permissible for products of animal, and plant products,
fishery products, F&B and wine products.
Global ICPP e-Phyto Hub: On July 7, 2020, pursuant to an agreement between APHIS and SENASA,
the U.S. and Argentina implemented an Electronic Phytosanitary Certification system for trade in plant
products. The e-phyto system was developed by the International Convention of Phytosanitary
Protection (ICPP) and benefits bilateral trade by reducing paperwork and providing a more efficient,
transparent, and reliable system.
Import fees: Due to persistent high inflation and the government’s power to alter fee structures with
little warning, exporters should work with an importer regarding the most current import fees or contact
Post for additional information.
Additional Information of Interest
FAO’s International Convention of Phytosanitary Protection (ICPP) recognized SENASA’s work on the
Control and Monitoring Program in Digital Media, which is published in its international guide and
shared with countries all over the world through its official website. The program aims to create a safer
and more reliable e-commerce environment and protecting on-line consumers of both animal and
vegetable products. The United States, Belgium, New Zealand, Canada, Jamaica, Denmark, and
Australia, together with Argentina, were selected by (ICPP) to participate in the “Guide for Pest Risk
Management” which Represent Products Requested through Digital Media and Distributed by Mail and
Messenger Services.”
In July 2023, ANMAT/INAL launched the SIFeGA (Sistema de Informacion Federal para la Gestion
del Control de los Alimentos – Federal Information System for Food Control Management) website. Its
primary goal is to improve access to clear and updated information. Easy access is facilitated to RNE
and RNPA registers, food handlers and trainers information, and the CAA and the Nutritional Seal and
Warning System as well (SIFeGA | Argentina.gob.ar).

26
Appendix I. Government Regulatory Agency Contacts
Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA)
Coordinación de Relaciones Internacionales
Avda. Paseo Colón 367, piso 5
Buenos Aires, Argentina
Tel: (54-11) 4121-5353
E-mail: [email protected]
Instituto Nacional de Alimentos (INAL)
Relaciones Internacionales
Estados Unidos 25
Buenos Aires, Argentina
Tel: (54-11) 4342-5674; 4340-0800
E-mail: [email protected]
Instituto Nacional de Vitivinicultura (INV)
San Martín 430
Mendoza, Argentina
Tel: (54-261) 5216600
Appendix II. Other Technical Import Contacts
Cámara de Importadores de la República Argentina (CIRA)
Avda. Belgrano 427, piso 7
Buenos Aires, Argentina
Tel: (54-11) 4342-1101
E-mail: [email protected]
Attachments:
No Attachments
