
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 1
From the Factory to the Frontlines
The Operation Warp Speed Strategy for Distributing a COVID-19 Vaccine
What This Strategy Aims to Do
This report to Congress details a strategy to achieve the principal purpose and objective of
Operation Warp Speed (OWS): ensuring that every American who wants to receive a COVID-19
vaccine can receive one, by delivering safe and eective vaccine doses to the American people
beginning January 2021.
The leadership of OWS has committed to being transparent with Congress, the media, and the
American people. OWS has provided regular briefings on topics of interest to Congress and the
media and will continue to provide updates and announcements as OWS reaches new milestones.
Congress has been a vital partner in the all-of-America response to the COVID-19 pandemic.
With support provided through emergency supplemental and flexible discretionary funding,
OWS has now made strong progress toward a safe and eective COVID-19 vaccine, with multiple
candidates in Phase 3 clinical trials.
Simultaneously, OWS and partners are developing a plan for delivering a safe and eective
product to Americans as quickly and reliably as possible. Experts from the Department of
Health and Human Services (HHS) are leading vaccine development, while experts from the
Department of Defense (DoD) are partnering with the Centers for Disease Control and Prevention
(CDC) and other parts of HHS to coordinate supply, production, and distribution of vaccines.
Successful implementation of the national COVID-19 vaccination program requires precise
coordination across federal, state, local, tribal, and territorial governments and among many
public and private partners. Cooperation on each of these fronts has already begun, as detailed
throughout this strategy document.
OWS is harnessing the strength of existing vaccine delivery infrastructure while leveraging
innovative strategies, new public-private partnerships, and robust engagement of state, local,
tribal, and territorial health departments to ensure ecient, eective, and equitable access to
COVID-19 vaccines.
Some variables that will impact the planning of this vaccination program are unknown
until a vaccine is authorized or approved by the Food and Drug Administration (FDA), such
as populations for whom a given vaccine is most appropriate, distribution and storage
requirements, dosage requirements, and other variables. This document lays out a flexible
strategy that can accommodate a range of scenarios.
Through the COVID-19 vaccination program, OWS seeks to achieve maximum uptake of the
vaccine across all population groups. The eventual objective of the vaccination program is to
leave the U.S. government and commercial infrastructure better able to respond to pandemics
and public health crises in the future.
From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 2
What Is the Strategy?
Once a vaccine has received approval or authorization from the FDA, the four key tasks to achieve
the primary objective of ensuring vaccine access for every American who wants it are to:
Continue engaging with state, tribal, territorial, and local partners, other stakeholders,
and the public to communicate public health information, before and after distribution
begins, around the vaccine and promote vaccine confidence and uptake.
Distribute vaccines immediately upon granting of Emergency Use Authorization/
Biologics License Application, using a transparently developed, phased allocation
methodology.
Ensure safe administration of the vaccine and availability of administration supplies.
Monitor necessary data from the vaccination program through an information
technology (IT) system capable of supporting and tracking distribution,
administration, and other necessary data.
This report lays out the requirements for each of these tasks and how OWS has taken action and
is planning future actions to execute on them.
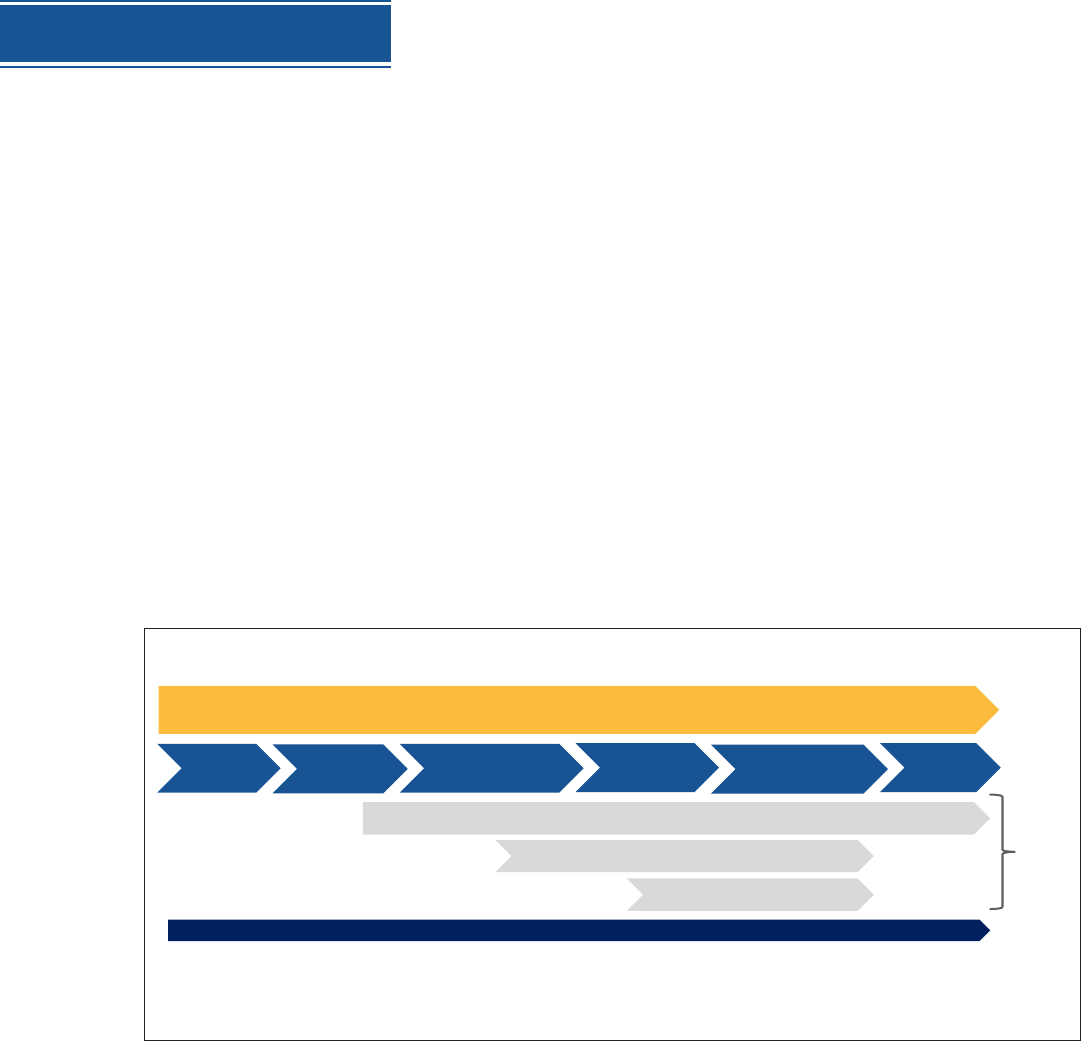
MULTIPLE CRITICAL COMPONENTS TO VACCINE IMPLEMENTATION
Multiple Critical Components to Vaccine Implementation
Supply - Monitor, Track, Report
Vaccine Uptake, Use, and Coverage
ADE and Vaccine Effectiveness
Monitoring and Reporting
Regulatory Considerations
Prioritizing
population
Allocation of
Vaccine
Distribution
(MFR –Dist- State)
Administration
Safety, Effectiveness,
Uptake, Second dose
Vaccine
Recovery
Communication and Stakeholder Guidance
(state, tribal, local, special populations, private sector partners, public)
Data
Public health impact relies on rapid, efficient, and high uptake
of complete vaccine series, with focus on high-risk groups

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 3
Distribution
What is required: A distribution plan must be able to deliver vaccines immediately upon FDA
authorization or licensure to all possible administration endpoints, while remaining flexible
enough to accommodate a variety of factors, including varying product requirements and
manufacturing timelines and volumes. Any distribution eort must ensure safety of the products,
maintain control and visibility, manage uptake and acceptance, ensure traceability of product,
and maximize coverage, which requires a centralized solution as well as close local partnerships.
What we are doing: OWS is developing a cooperative plan for centralized distribution that will
be executed in phases by the federal government, the 64 jurisdictions CDC works with (all 50
states, six localities, and territories and freely associated states), Tribes, industry partners, and
other entities.
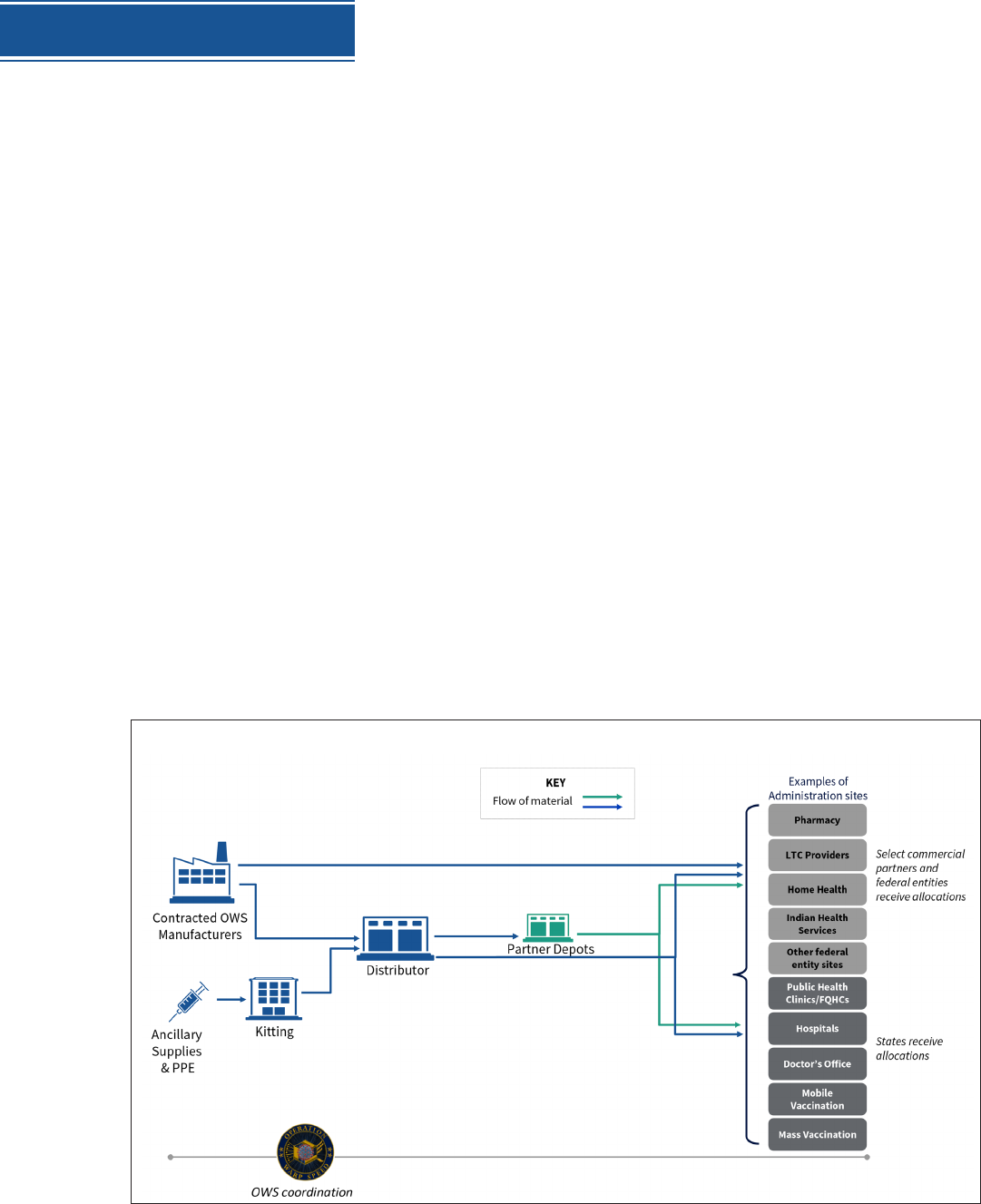
Distribution has three key components:
Partnerships with state, local and tribal health departments, territories, Tribes,
and federal entities to allocate and distribute vaccines, augmented by direct
distribution to commercial partners.
A centralized distributor contract with potential for back-up distributors for
additional storage and handling requirements.
A flexible, scalable, secure web-based IT vaccine tracking system for ongoing
vaccine allocation, ordering, uptake, and management.
State, Tribal, and Local Partnerships
CDC is working with state, local and tribal health departments to hone existing plans for vaccine
distribution and administration. CDC has worked for decades with these partners, including
under cooperative agreements, to ensure public health systems are prepared with plans,
trained personnel, strategic relationships and partnerships, data systems, and other resources
needed for sustaining a successful routine immunization infrastructure, and these plans will
be adapted for this vaccine program.
CDC awarded grants as part of the Coronavirus Aid, Relief, and Economic Security (CARES)
Act and the Families First Coronavirus Response Act that can help immunization programs
begin preparation for vaccine distribution and administration. The funding will be used to
enhance capacity to support stang, communication and stakeholder engagement, pandemic
preparedness, and mass vaccination.
A multi-agency federal team has worked with five pilot jurisdictions—California, Florida,
Minnesota, North Dakota, and Philadelphia—to utilize a basic plan for administration and
adapt it to create jurisdiction-specific plans that will serve as models for other jurisdictions.
Jurisdiction planning will cover coordination with federal facilities in their jurisdiction,
coordination with national chain partners, vaccination of critical work forces, and reaching
underserved populations.
Each jurisdiction will be required to develop a “microplan,” based on their existing plans as well
as outputs from the first five jurisdictions supported, with CDC providing technical assistance.
These microplans will identify vaccination sites and necessary logistical considerations and
lay out how the sites will be onboarded into the necessary IT system. The microplans will need
to be flexible to allow adaptation as more information about the specific characteristics of the
vaccines becomes available.

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 4
Under their cooperative agreements with CDC through which CARES Act awards were made,
jurisdictions will then onboard providers to the IT system and identify and plan for the necessary
vaccination workforce. Jurisdictions will also be responsible for laying specific groundwork for
vaccinating high-risk and prioritized populations through various outreach eorts, including
a work group or stakeholder groups, and forming a vaccination committee.
Jurisdictions will be expected to incorporate planning for distribution of vaccines to mem-
bers of Tribes into their microplans. In addition, CDC and OWS are working with the Indian
Health Service (IHS) to develop a plan for direct IHS distribution of vaccine to Tribes that
desire that option.
Centralized Distribution
Centralized distribution allows the government full visibility, control, and ability to shift
assets and use data to optimize vaccine uptake. On August 14, CDC announced its centralized
distributor contract by executing an existing contract option with McKesson, which distributed
the H1N1 vaccine during the H1N1 pandemic in 2009–2010. The current contract with McKesson,
awarded as part of a competitive bidding process in 2016, includes an option for the distribution
of vaccines in the event of a pandemic.
Once vaccines are allocated to a given jurisdiction or authorized partner, McKesson will deliver
a specific amount of vaccine to a designated location. In many instances, delivery locations will
be sites where vaccine will be administered. Alternatively, vaccines can be delivered to locations
in jurisdictions to be further distributed to administration sites within health department
networks. Vaccines can also be delivered to locations integrated into national retail pharmacy
networks for distribution to individual pharmacies.
This system will be scalable to meet demand. Some vaccine with ultra-cold storage requirements
may be shipped directly from the manufacturer to the administration sites, but all distribution
will be managed by this centralized system.
If necessary, the McKesson contract can cover rapid distribution of doses of refrigerated (2–8º
Celsius) and frozen (-20ºC) vaccines.
The COVID-19 pandemic has likely accelerated a trend towards dierent ways of engaging with the
healthcare system, and successful delivery of this vaccine will need to incorporate new types of
sites and approaches for vaccine delivery. For example, during H1N1, once vaccines became widely
available pharmacies played an important role in the vaccine distribution; pharmacies’ role is even
more critical to vaccinations today and will be fully integrated into the distribution plan.
Ordering and Tracking Systems
Vaccine allocation and centralized distribution will utilize HHS’s Vaccine Tracking System
(VTrckS), which is a secure, web-based IT system that integrates the entire publicly funded
vaccine supply chain from purchasing and ordering through distribution to participating state,
local, and territorial health departments and healthcare providers.
VTrckS is being scaled for distribution of pandemic vaccines, to include the onboarding of
new providers under each jurisdiction’s microplan. For the COVID-19 vaccination program,
additional providers, including private partners (e.g., pharmacy chains) and other federal
entities (e.g., the Indian Health Service), will be onboarded to enable allocation to and ordering
directly by these partners, in addition to the state, local, and territory allocations.
Through the linkage of a number of systems, information technology will also help direct
people to where to get vaccinated using web-based “finder” systems.
From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 5
A Potential Phased Structure
Phase 1: Upon FDA authorization or approval, initial vaccine doses will be distributed in a fo-
cused manner, with the goal of maximizing vaccine acceptance and public health protection
while minimizing waste and ineciency.
Although final decisions about prioritization will not be made until closer to implementation,
select scenarios have been developed to assist with state and local planning. State and local
health departments have been given specific scenarios to plan for during this stage, while sce-
nario planning for distribution and administration plans specific to focused populations has
begun at the federal level.
Phase 2: As the volume of available vaccine increases, distribution will expand, increasing ac-
cess to the larger population. When larger quantities of vaccine become available, there will be
two simultaneous objectives: 1) to provide widespread access to vaccination and achieve coverage
across the United States population and 2) to ensure high uptake in target populations, particu-
larly those who are at high risk for severe outcomes from COVID-19.
Phase 3: If the risk of COVID-19 persists such that there remains a public health need for an
ongoing vaccination program, COVID-19 vaccines will ultimately be universally available and
integrated into routine vaccination programs, run by both public and private partners.
Based on the timeline associated with FDA regulatory decision-making, increasing quantities
of produced vaccines may be stockpiled as manufacturing proceeds before a regulatory decision
has been made, which would mean that distribution may begin directly with Phase 2 or Phase 3.
Allocation: Allocations in the early phases will be based in part on methodology previously de-
veloped and reviewed by public health experts as part of pandemic planning. This methodology
will be adjusted based on experience from COVID-19, real-time data on the virus and its impact
on populations, performance of each vaccine, and the ongoing needs of the essential workforce.
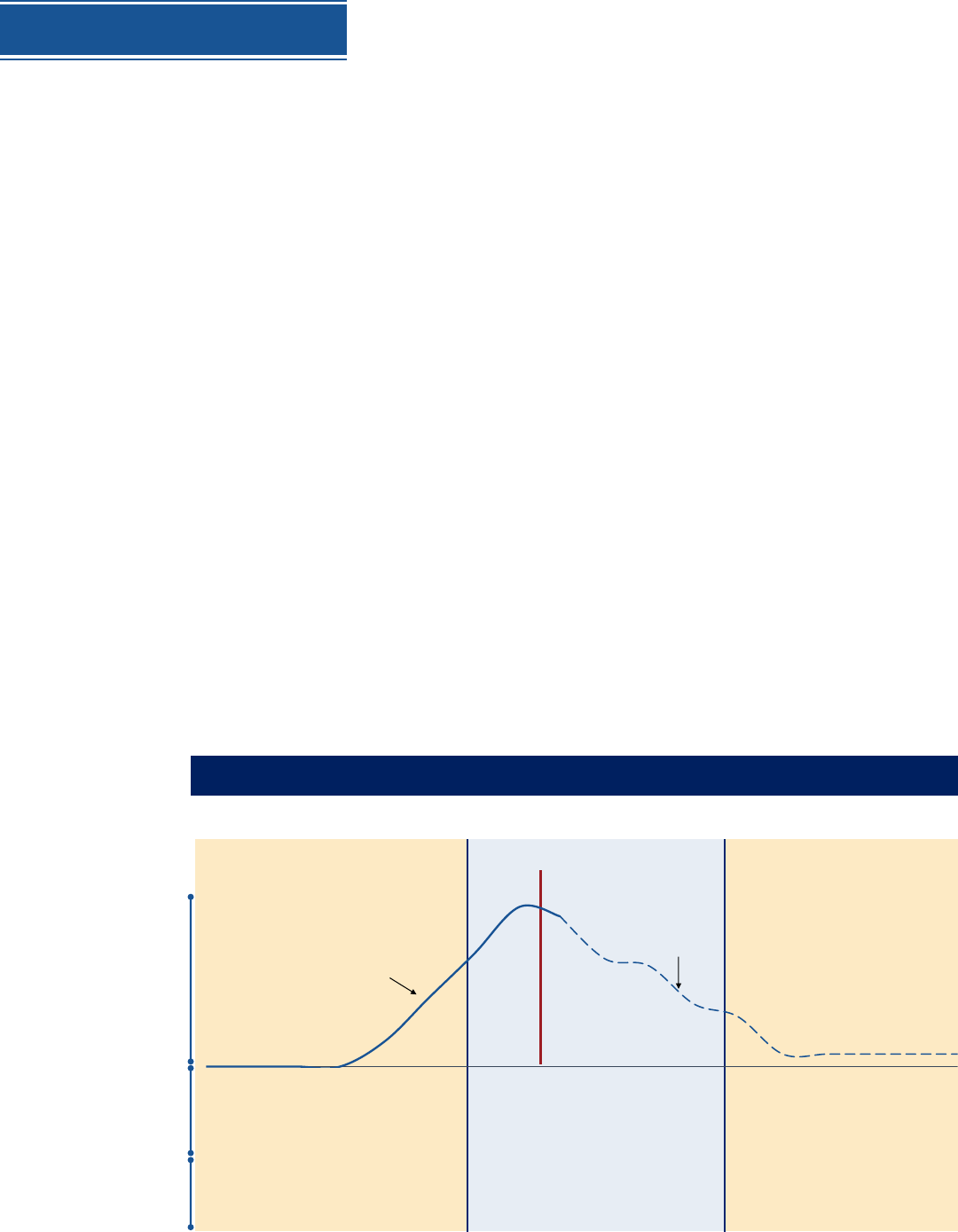
Distribution will adjust as volume of vaccine doses increases,
moving from targeted to broader populations reached (phased approach)
Limited Doses Available Large Number of Doses Available
Continued Vaccination,
Shift to Routine Strategy
• Constrained supply
• Highly targeted administration required to
achieve coverage in priority populations
• Likely sufficient supply to meet demand
• Supply increases access
• Broad administration network required
including surge capacity
• Likely excess supply
• Broad administration network for
increased access
• Tightly focus administration
• Administer vaccine in closed settings (places
of work, other vaccination sites) specific to
priority populations
• Expand beyond initial populations
• Administer through commercial and private
sector partners (pharmacies, doctors offices,
clinics)
• Administer through public health sites (mobile
clinics, FQHCs, targeted communities)
Doses available per month
(baseline as of 07/16)
Illustrative scenario for planning purposes; will be adapted based on the clinical / manufacturing
information on all OWS candidates and vaccine prioritization
~660M cumulative
doses available
Illustrative ramp-down, not
based on OWS decisions or
candidate projections
• Open vaccination
• Administer through commercial and
private partners
• Maintain PH sites where required
Volume
doses
available
(per month)
Max
Trials only
Key
factors
Likely
admin
strategies

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 6
To develop and update populations to target in settings with limited doses of vaccine, the
National Institutes of Health (NIH) and CDC requested that the National Academies of Sciences,
Engineering, and Medicine and the National Academy of Medicine (NAM) develop an overarching
framework to assist policymakers in the U.S. and global health communities in planning for
equitable allocation of vaccines against COVID-19.
NAM established a committee to consider the criteria that should be used to set priorities for
equitable distribution of potential vaccine and released a discussion draft of a preliminary
allocation framework on September 1. The findings from the NAM committee will be shared
with the CDC’s Advisory Committee on Immunization Practices (ACIP), to help inform the
committee’s deliberations related to vaccine priority groups and ensuring equity in vaccination
in the United States.
ACIP will review evidence on COVID-19 epidemiology and burden, vaccine safety, vaccine
ecacy, evidence quality, and implementation issues to inform recommendations for
COVID-19 vaccine policy, including priority groups for vaccination, which are submitted to
the CDC director for adoption. ACIP meetings are open to the public, and committee records
are required to be made available to the public, ensuring transparency and visibility for this
recommendation-making process.
ACIP formed a COVID-19 Vaccine Work Group to help inform its evidence-based approaches to
COVID-19 vaccination policy, including the initial vaccine prioritization strategy to be presented
to the full ACIP for deliberation at public ACIP meetings, development of recommendations, and
eventual presentation of these recommendations to the CDC for consideration in determining
population prioritization.
ACIP embarked on early planning for these eorts. The framework developed during, and the
lessons learned from, the H1N1 influenza vaccine implementation are being used to guide
COVID-19 vaccine prioritization. CDC learned several lessons from the H1N1 response and
vaccine distribution, including the real possibility of uncertainties in the pharmaceutical
manufacturing process, which requires the distribution plan to anticipate delays and respond to
changing circumstances. Further, demand is likely to vary regionally and in diverse populations
within a given geographic area. Nimble delivery and allocation strategies will be essential.

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 7
Administration
What is required: Successful administration requires identifying prioritized populations and
working cooperatively with state, local and tribal public health departments and other key
partners to ensure individuals in targeted groups safely receive vaccines when limited doses
initially become available.
What we are doing: Through collaborative planning with states and private sector provider
partners such as pharmacies, vaccine administration sites will be selected to optimize access
to vaccines throughout the distribution process.
Administration tasks within each distribution phase will include:
Delivery of vaccine to sites, with the goal of no upfront costs to providers and no out-
of-pocket cost to the vaccine recipient.
Ensuring administration sites, as covered in the jurisdiction’s microplans, have the
capabilities for storing, handling, and administering vaccine products with specific
distribution and administration requirements.
Supporting reliable distribution of ancillary supplies that may be necessary for vaccine
administration.
Engagement of traditional and non-traditional administration sites and approaches in
vaccination planning to allow for flexibility to accommodate vaccine requirements.
Delivery and Cost
The federal government is procuring hundreds of millions of doses of safe and eective vaccines,
and has contracted with McKesson for purposes of vaccine distribution, such that no American
will be charged for either the COVID-19 vaccine or its distribution. Various plans, supported
by the CARES Act and the Families First Coronavirus Response Act, are under development
with the objective of ensuring no one will be charged any out–of-pocket expenses for the
administration of the vaccine either. The objective is to ensure no one desiring vaccination will
face an economic barrier to receiving one.
Section 3203 of the CARES Act (P.L. 116-136) requires health insurance issuers and plans to
cover any ACIP-recommended COVID-19 preventive service, including vaccines, without cost-
sharing within 15 days of such recommendation to the CDC. Once a licensed COVID-19 vaccine
is recommended by ACIP, and the recommendation is adopted by the CDC Director, required
coverage for vaccines as preventative services for Medicaid Early and Periodic Screening,
Diagnostic and Treatment beneficiaries and the Aordable Care Act provisions for most private
insurance coverage and for the Medicaid expansion populations will also apply.
Ancillary Supplies
Supporting and securing an adequate quantity of ancillary supplies needed for administration
has been a collaborative, interagency eort. OWS has aimed to procure and assemble 6.6 million
ancillary supply kits, including pediatric, adult, and mixed-use kits, which would support the
vaccination of up to 660 million doses of vaccine. These kits will include needles, syringes,
alcohol pads, vaccination cards, and limited PPE for vaccinators.
HHS’s Biomedical Advanced Research and Development Authority (BARDA) has awarded four
large task orders for needles and syringes. BARDA will support additional solicitations, in
coordination with the Strategic National Stockpile, to maximize the availability of needles
From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 8
and syringes toward the end of 2020. BARDA and the DoD Joint Program Executive Oce for
Chemical, and Biological, Radiological, and Nuclear Defense (JPEO-CBRND) have awarded
three agreements to increase needle and syringe capacity in the U.S. for the future, some of
which will be available in time to support the COVID-19 vaccination in early 2021. BARDA and
the JPEOCBRND have also awarded agreements with two domestic manufacturers of vials to
increase capacity necessary to support multiple vaccine candidates.
Administration Sites
Administration site options will vary depending on the nature of the vaccine and the phase of
the vaccination program. During Phase 1, administration sites may be more limited to settings
that can optimize reaching the target population while meeting the early requirements
for storage and handling of vaccine product. During Phase 2, an expanded administration
network would, for instance, likely include adult and pediatric healthcare providers and
pharmacies. These considerations will be part of planning done by the jurisdictions discussed
in the Distribution section.
As part of eorts to make administration sites easily accessible, the program will make
maximum use of all healthcare professionals licensed to administer vaccines, including allied
health professionals such as pharmacists.
HHS is also committed to ensuring rural populations can receive the vaccine, and has decades
of experience working with public health partners addressing the needs of hard-to-reach
populations. CDC will work with local communities, governments, and other partners to
identify the best places and times to reach this population and utilize strategic distribution
points via community health centers, schools, workplaces, mobile clinics, and pharmacies.
OVERVIEW OF DISTRIBUTION AND ADMINISTRATION

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 9
Monitoring
What is required: The vaccination program requires extensive data monitoring infrastructure,
including appropriate IT architecture, to incorporate claims and payment processes, to identify
when a person needs a potential second dose, to monitor outcomes and adverse events, and to
account for products the U.S. government is spending billions of dollars to research, develop,
and produce. Data will need to be available both federally and at the state, local, and tribal level
to ensure ecient management of the vaccination program.
What we are doing: OWS will construct and integrate an IT architecture that achieves this
objective, building o of existing IT infrastructure and filling gaps with new IT solutions.
CDC has already been working to improve the data infrastructure needed to better track
vaccines, vaccination, and related information. The COVID-19 vaccination program requires
significant enhancement of the IT that will support enhancements and data exchange that are
critical for a multi-dose candidate to ensure proper administration of a potential second dose.
Immunization Information Systems used by state, territory, and city entities that deliver
public vaccinations will be central to this IT infrastructure. Major pharmaceutical retailers
have proven and reliable dispensing record systems, while healthcare systems, hospitals, and
private providers employ Electronic Health Record systems to store, monitor, and track patient
information. Points of administration with undeveloped infrastructure—such as ad hoc mobile
clinics and other rapidly mobilized mass vaccination sites—will be provided with free access
and training for purpose-built web-based applications to support vaccine data administration
and tracking, with an array of options available to make these accessible.
Together, this data will be reported into a common IT infrastructure that will support analysis
and reporting. The IT infrastructure will support partners with a broad range of tools for
record-keeping, data on who is being vaccinated, and reminders for second doses.
In all cases, administration records will be aggregated, anonymized, and de-identified to
protect personally identifiable, private health information to the maximum extent possible.
Before a vaccine is authorized for use, evidence of its safety and ecacy is limited to the
results from clinical trials, where patients are selected carefully and followed up very
closely under controlled conditions. Because some technologies have limited previous data
on safety in humans, the long-term safety of these vaccines will be carefully assessed using
pharmacovigilance surveillance and Phase 4 (post-licensure) clinical trials.
The key objective of pharmacovigilance is to determine each vaccine’s performance in real-life
scenarios, to study ecacy, and to discover any infrequent and rare side eects not identified
in clinical trials.OWS will also use pharmacovigilance analytics, which serves as one of the
instruments for the continuous monitoring of pharmacovigilance data. Robust analytical tools
will be used to leverage large amounts of data and the benefits of using such data across the value
chain, including regulatory obligations. Pharmacovigilance provides timely information about
the safety of each vaccine to patients, healthcare professionals, and the public, contributing to
the protection of patients and the promotion of public health.

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 10
Engagement
What is required: To support vaccine distribution, administration, and monitoring, as well
as promote vaccine uptake, vaccine confidence, and reporting of adverse events, a successful
vaccination program requires engaging a nationwide network of partners. Working with
established partners—especially those that are trusted sources for target audiences—is critical
to advancing public understanding of, access to, and acceptance of eventual vaccines.
What we are doing: To build partnerships as part of the vaccination program and deliver an
eective communications strategy, OWS is engaging public, nonprofit, and private partners,
while leveraging the government’s longstanding relationships with state health departments,
tribal nations and organizations, healthcare systems, the vaccine industry, health insurance
issuers and plans, and non-traditional partners.
Partnerships
State, local and tribal health departments have conducted pandemic vaccination planning with
immunization and preparedness funding from CDC for over a decade. Rapidly updating these
vaccination response plans for COVID-19 will ensure readiness for timely administration of
COVID-19 vaccines.
This work builds on existing successful partnerships: Each year, CDC safely distributes more
than 80 million doses of vaccines to approximately 40,000 public and private health providers
across the country, in addition to the tens of millions of other vaccines distributed through other
channels. During the 2009 H1N1 pandemic, more than 70,000 provider sites participated in the
expanded vaccination program. This represents strong baseline capacity and partnerships for
distribution and administration.
HHS’s Oce of Intergovernmental and External Aairs has established communication
channels with almost 30 private sector organizations representing hospitals, physicians,
nurses, nursing homes, community health centers, health insurance issuers and plans, drug
stores, influencers, foundations, patients, and seniors’ groups to provide regular updates on
the work of OWS, including the distribution program.
HHS has also been holding regular calls with intergovernmental partners at the state,
local, tribal, and territorial levels, with robust dialogue on how the federal government will
successfully partner with them on the vaccination program.
Further, work has begun with organizations representing minority populations and vulnerable
communities, with consultation already occurring with more than 150 organizations dedicated
to addressing health disparities. Faith-based and other trusted community organizations can
also be critical in addressing vaccine hesitancy, and HHS’s Center for Faith and Opportunity
Initiatives is working with minority-serving faith and community groups to enlist their help in
educating Americans and encouraging participation in the vaccination program.
Communications
Strategic communications and public messaging are critical to ensure maximum acceptance of
vaccines, requiring a saturation of messaging across the national media.
An information campaign led by HHS’s public aairs department—developed using human-
centered design, extensive public and stakeholder engagement, and research on message
development and delivery—will focus on vaccine safety and ecacy, and target key populations
and communities to ensure maximum vaccine acceptance.

From the Factory to the Frontlines
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES | PAGE 11
CDC and other HHS components are working collaboratively within OWS to ensure that
consistent and accurate information is at the foundation of the communications eort. The
plan will also help inform the American people about the OWS strategy of delivering faster
results while still following the same processes for safety and eectiveness that Americans
expect with any other vaccine.
Identifying the right messages to promote vaccine confidence, countering misinformation, and
targeting outreach to vulnerable and at-risk populations will be necessary to achieve high cov-
erage. CDC will build on its existing relationships with local public health partners and health
departments to eectively implement communications, and CDC is also working to develop in-
novative approaches to improve vaccine uptake among hard-to-reach critical populations.
Understanding that public confidence in vaccines is necessary for vaccine uptake and accep-
tance, CDC will make use of its strategic framework, Vaccinate with Confidence, which it has
used successfully to strengthen public trust in vaccines and prevent vaccine-preventable dis-
ease outbreaks. This framework emphasizes three key priorities: protect communities, em-
power families, and stop myths. Within this framework, CDC is already working with local
partners and using trusted messengers to establish new partnerships and contain the spread
of misinformation.
